
Surgical procedure is the branch of medical practice that treats injuries, diseases, and deformities by the physical removal, repair, or readjustment of organs and tissues, often involving cutting into the body. Surgical devices are tools used to carry out the desired operation during the surgical procedure.
The devices can be organized based on the specialty of surgery like Bariatric Surgery, Breast Surgery, Cardiothoracic Surgery, Colon & Rectal Surgery, Craniofacial surgery, Endocrine Surgery, General Surgery, Gynecological Surgery, Hand Surgery, Head & Neck Surgery, Hernia Surgery, Neurosurgery, Oral and maxillofacial surgery, Orthopedic Surgery, Ophthalmological Surgery, Oncological surgery, Pediatric Surgery, Plastic & Reconstructive Surgery, Transplant Surgery, Thoracic Surgery, Trauma Surgery, Urologic Surgery (urinary tract system and reproductive organs) and Vascular Surgery.
The instruments can be categorized based on their functionality, such as handheld devices (cutting, dissecting, clamping, retracting, holding, grasping, examining, suturing, stapling, suction and aspiration devices), access devices (endoscope, trocars), energy devices (ultrasound, bipolar).
Based on the size of the incision, the surgical procedure can be:
Open surgery means the cutting of skin and tissues so that the surgeon has a full view of the structures or organs involved. (Figure 1)
Minimally Invasive Surgery
This is a technique of surgery that does not need a large incision and is performed using small incisions or cuts and fewer stitches. This approach allows the patient to recuperate faster with less pain. (Figure 1)
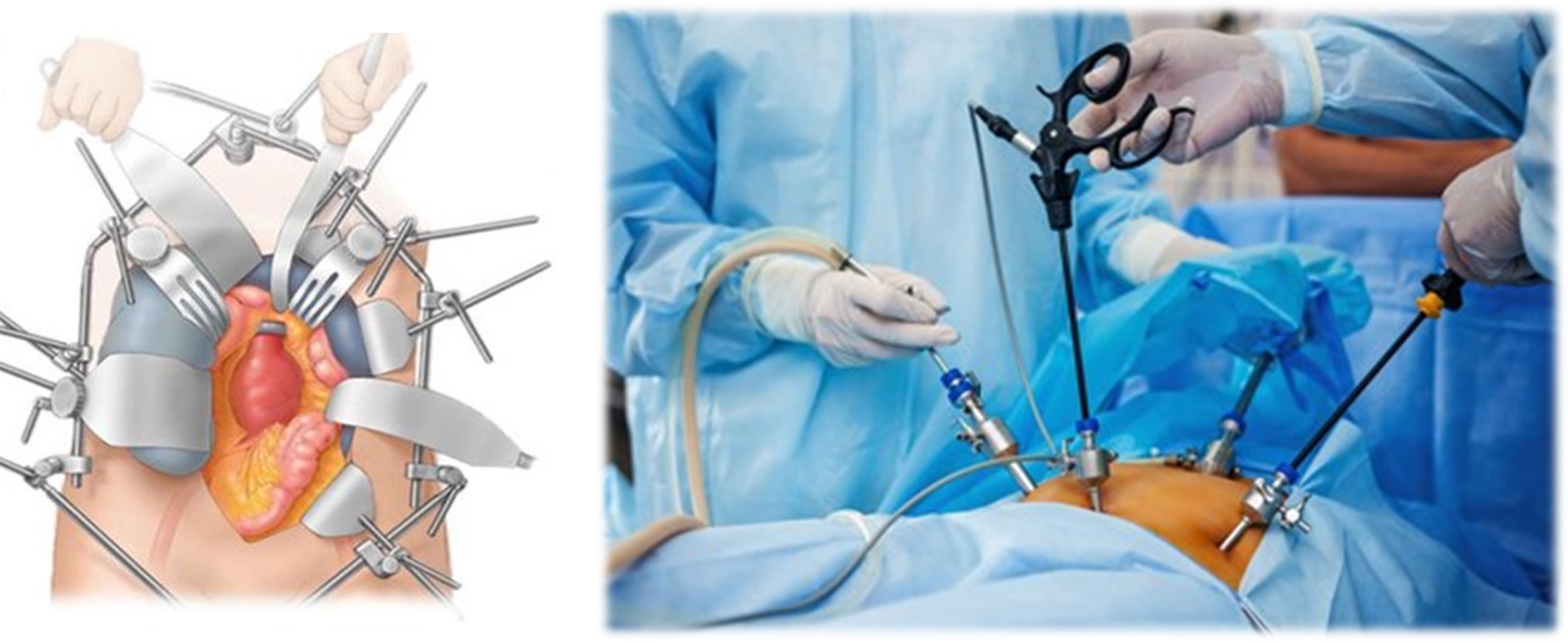
Figure 1 (Left) Open Surgery (Right) Minimally invasive surgery
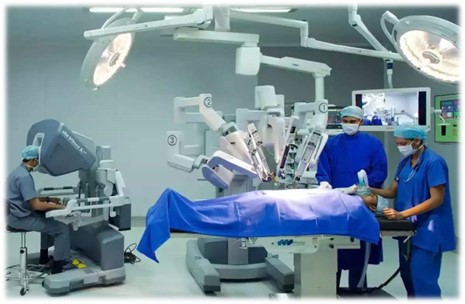
Robotic Surgery – This is a form of minimally invasive surgery, that allows doctors to perform many types of complex procedures with more precision, flexibility, and control, utilizing a surgical platform where surgical instruments are attached to mechanized arms and operated from the console. (Figure 2)

Figure 2 Robotic Surgery
The devices used will vary based on the type, specialty, and functionality at that instance of the surgical procedure.
Factors Taken into Consideration While Formulating Standards for Surgical Devices:
“A Workman who wants to do his work must first prepare his tools”. Well, this applies to surgeons and surgical instruments included. Therefore, it is vital to ensure
• Suitable material composition
• With appropriate mechanical properties
• Magnetic properties
• Performance characteristics
• Biocompatibility requirements
• Ergonomic properties
• Sterility requirements
MEDICAL-GRADE SURGICAL MATERIALS
Immense care has been taken to prescribe the material used for the manufacturing of surgical devices, taking into account considerations like compatibility, hardness, etc., as choosing the wrong material lead to disastrous consequences. Currently, Stainless steel, Titanium, Tantalum, Platinum, and Palladium are widely in use.
INDIAN STANDARDS FOR SURGICAL DEVICES
The Indian Standards for Surgical Instruments are formulated under the Surgical Instruments Sectional Committee MHD 01. The Nomenclature of the Surgical Instruments follows Universal Medical Devices Nomenclature System (UMDNS), a coding system used to generically classify and identify medical devices. The Standards formulated by MHD 01 are consolidated in the Program of work of the committee.
BIBLIOGRAPHY & REFERENCES
[1] Program of Work (POW) of MHD 01 Surgical Instruments Sectional Committee https://www.services.bis.gov.in/php/BIS_2.0/bisconnect/pow_new
[2] Universal Medical Device Nomenclature SystemTM (UMDNS) https://www.ecri.org/solutions/umdns
[3] Mastery of Endoscopic and Laparoscopic Surgery. (2013). United States: Wolters Kluwer Health.
[4] Differentiating Surgical Instruments. (2011). United States: F.A. Davis.
[5] Mishra, R. (2013). Textbook of Practical Laparoscopic Surgery. India, Jaypee Brothers Medical Publishers Pvt. Limited.